Oxidation Chemistry: Chemistry of Reactive Oxygen Species with Organometallic Compounds and Heteroatoms
The Selke group uses a variety of methods (time-resolved spectroscopy, steady-state kinetics, as well as preparative methods) to study the chemistry of reactive oxygen species, especially singlet dioxygen. This area is of interest in biology as well as catalyses.
Light and oxygen can be toxic. Cell constituents or certain dyes found in the environment (some plant pigments, flavins etc.) absorb light and subsequently interact with oxygen molecules causing the latter to become more reactive. This process is called photosensitization. The oxygen molecule may then react with DNA constituents, amino acids, and with some amino acids coordinated to metals found in enzymes. This process may damage or destroy the enzyme. On the other hand, the same process is used in tumor phototherapy, where tumor cells are killed as described above. Understanding the chemistry of this process may therefore be of medicinal importance. One of the oxygen species produced during the sensitization process is singlet oxygen. Reactions of singlet oxygen with biomolecules have been studied for several decades, but its chemistry with organometallic compounds and heteroatoms has received far less attention.
The major research focus of our group is the exploration of the chemistry of singlet oxygen with organometallic compounds and compounds containing heteroatoms such as phosphorus and sulfur. The basic aims are threefold: (i) To solve a number of fundamental mechanistic questions in oxidation chemistry, (ii) to find new oxidants derived from the most benign oxidant, i.e. molecular oxygen, and (iii) to develop new photosensitizers for the production of singlet oxygen with potential biomedical applications.
Reactive intermediates in oxygen chemistry are often difficult to detect because they themselves are powerful oxidants. We are trying to find new methods (using singlet dioxygen) to observe and possibly isolate such species and, where applicable, to investigate their potential as oxidants. We are also exploring the susceptibility of metal-bound amino acids (especially cysteine) and thiolate ligands on some metallic nanoparticles towards oxidation by singlet oxygen.
We are also investigating organometallics compounds both as quenchers and sensitizers for the production of singlet oxygen, and we are exploring use of nanomaterials as carriers for singlet oxygen sensitizers.
To learn more about some of the chemistry in the Selke group, click on the following tabs.
What is Singlet Oxygen?
Singlet oxygen is the lowest excited state of the dioxygen molecule. Its lifetime in solution is in the microsecond range (3 µsec in water to about 700 µsec in C6D6). It undergoes several reactions with organic molecules (Ene-Reaction, Diels-Alder Reaction). These reactions have been studied for many years.
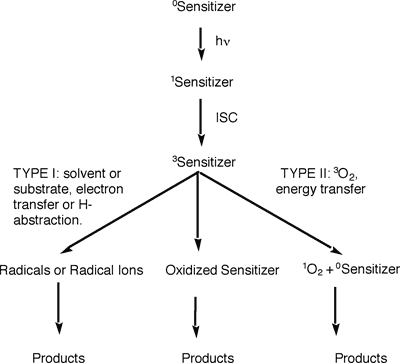
In solution, the singlet oxygen is often prepared by a process called photosensitization. A photosensitizer is irradiated to its singlet excited state, followed by conversion (called intersystem crossing) to its triplet excited state. The triplet excited sensitizer may undergo radical reactions (Type I process) or produce singlet oxygen (Type II process). Superoxide formation (another reactive oxygen species) is also possible, but rare. The type II process is usually preferred at low substrate concentration and high oxygen concentration.
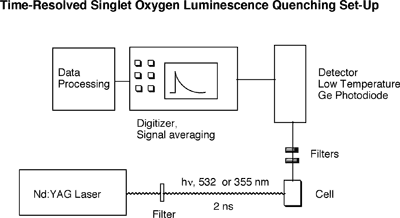
During its decay back to the ground state, the singlet oxygen molecule emits some radiation in the near IR region. This luminescence is very weak, but can be detected. The Selke group uses a low-temperature GE photodiode to directly observe singlet oxygen luminescence. When a fast laser pulse (nanosecond range) is used to excite the sensitizer, the decay of the singlet oxygen molecule can be directly observed over time. A photo and a block diagram of this time-resolved set-up are below.
This time-resolved set-up allows direct determination of rate constant by which singlet oxygen is consumed. This rate constant is the sum of quenching by solvent (kd), physical quenching(kq), and reaction of substrate (kr). kobsd = kd + [Substr.] (kq + kr)
Chemistry of Arylphosphines with Singlet Dioxygen
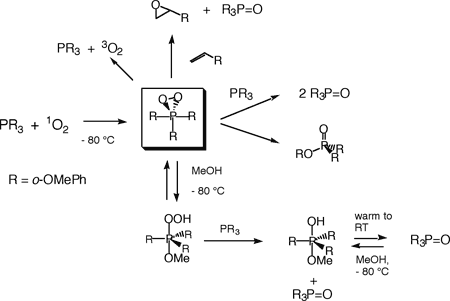
We are exploring the chemistry of arylphosphines with singlet oxygen. The main goal of this work was to directly observe the primary intermediates of this reaction, and to investigate the oxidizing power of these peroxidic species. Such peroxidic intermediates are even better oxidizing agents than singlet oxygen itself and therefore rapidly react with unreacted starting material. It is therefore not surprising that such species are very difficult to observe. To prevent (or at least slow down) reaction of intermediates with starting material, we have been studying arylphosphines possessing substituents in the ortho position of the aromatic rings. At very low temperature (- 80 °C), we have been able to directly observe and study several phosphadioxiranes. These compounds are indeed powerful oxidants: They epoxidize olefins, and intramolecular hydroxylation of an aromatic ring has also been observed by our group. For a recent review article in Chemical & Engineering News, click here. (Chemical & Engineering News 2003, 81(14), 28-31.) Several other unprecedented species obtained under protic conditions have also been characterized. The various reactions of a phosphadioxirane are shown below. Several other phosphines are currently under investigation.
Chemistry of Metal-Bound Thiolato Ligands with Singlet Oxygen
Metal-bound thiolato ligands are ubiquitous in many different areas of chemistry, ranging from metal-bound cysteine in metalloproteins to surface-capping ligands on metallic nanoparticles.
We have been investigating mechanistic issues surrounding fundamental questions concerning the mechanism, potential reaction pathways, and susceptibility of thiolato ligands toward oxidation. Some fundamental questions we seek to answer: How susceptible to oxidation are metal bound cysteine ligands? What factors (i.e. type and oxidation state of the metal, availability of coordination sites, etc.) control this reactivity? What are the products of such reactions and do the products remain coordinated to the metal?
We have found that singlet oxygen reacts with with a variety of metal thiolato complexes, ranging from Cobalt complexes with N,S-bound cysteine ligands to various Platinum(II)thiolato complexes, and Cadmium-sulfur clusters with phenyl thiolato ligands. In the latter case both physical quenching of singlet oxygen and photooxidation of the cluster are observed.
Bis-Cyclometallated Ir(III) Complexes as
New Singlet Oxygen Sensitizers
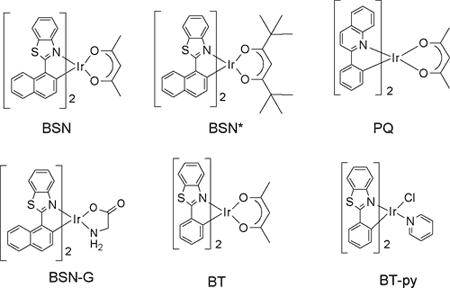
In solution, singlet oxygen is usually prepared by using various sensitizers (a dye or pigment, a quinone or an aromatic compound, examples are Methylene Blue, Rose Bengal, C60). The excited sensitizer, usually in the triplet state, reacts with triplet oxygen to give singlet oxygen. Some organometallics are also photosensitizers for singlet dioxygen, but overall, there has been relatively little work in this area. We are examining the photophysical properties of the bis(cyclometallated) Ir(III) and Pt(II) complexes prepared by the group of Prof. Thompson at the University of Southern California. We have found that these complexes are excellent sensitizers for the production of singlet oxygen. The bis-cyclometallated Ir(III) complexes (BT)2Ir(acac), (BSN)2Ir(acac), and (PQ)2Ir(acac); BT = 2-phenylbenzothiazole, BSN = 2-(1-naphthyl)benzothiazole, PQ = 2-phenylquinoline, and acac = acetylacetonate) studied are depicted below. Complexes with acetylacetonate ancillary ligands give singlet oxygen quantum yields near unity (FD = (0.7 - 1.0) ± 0.1)., whether exciting the ligand-based state or the lowest energy excited state (MLCT + 3LC). Similar complexes were prepared with amino acids (glycine) or pyridine tethered to the Ir(III) center (i.e. BSN-G, and (BT-py; G =glycine, and py = pyridine).
One disadvantage for use of these complexes in photodynamic therapy is the fact that they generally absorb from the near UV range to about 520 – 560 nm. This is not an ideal range as far as application for photodynamic therapy is concerned. Human skin has an absorption minimum near 800 nm. There are no good sensitizers that absorb near 800 nm, despite decades of efforts to prepare such sensitizers. Addition of suitable substituents to the ligands may allow significant red-shifting of the absorption maxima of these sensitizers, thereby making them interesting candidates for photodynamic therapy.